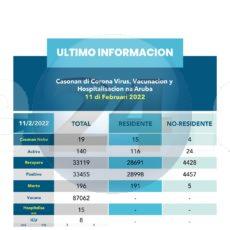
| Washington, D.C., 23 December 2021 (PAHO) – The Pan American Health Organization (PAHO) welcomed the WHO listing today of an AstraZeneca vaccine jointly produced by Argentina and Mexico – the first such decision for a COVID-19 vaccine manufactured in Latin America. The vaccine, with the international denomination COVID-19 Vaccine (ChAdOx1-S [recombinant]), is jointly manufactured by Argentina’s mAbxience, which reproduces its active pharmaceutical ingredient, and Mexico’s Laboratorios Liomont, which mixes and finishes the product for distribution. “We applaud this news,” the PAHO Director Carissa F. Etienne said. “This is an important milestone for Latin America and highlights the importance of technology transfer to increase the availability of quality COVID-19 vaccines in the region.” The regionally produced AstraZeneca vaccine is already in use in some Latin American and Caribbean countries, and inclusion in the World Health Organization (WHO) Emergency Use Listing will facilitate its procurement and distribution through PAHO’s Revolving Fund as well as COVAX, the international mechanism to increase global COVID-19 vaccines access. The approval comes as the pandemic enters its third consecutive year and the region sees a steady climb in COVID-19 cases. In the past week, the Americas reported over 1.1 million new COVID-19 infections – a 6% increase in cases from the previous week. Vaccine inequity, however, continues to divide the region, with a handful of countries unlikely to reach the 40% vaccination target by the end of the year and many just above the 50% threshold of full COVID-19 immunization. Boosting regional capacity to produce vaccines is key to bridging this gap, the PAHO Director said, citing the international endorsement as an example of how the region is primed to develop its pharmaceutical manufacturing capacity. “If given the opportunity and tools, our region can deliver,” she stressed. PAHO recommends countries opt for WHO Emergency Use Listing vaccines, which are evaluated based on international standards for quality, safety and efficacy. In an effort to expand access to such vaccines in Latin America and the Caribbean, PAHO provided support to the regulatory authorities in the two countries to fulfill the WHO listing requirements. The PAHO Director added, however, that the milestone was achieved thanks to the commitment of the public and private sectors in Argentina and Mexico, especially “investments made in the development of a scientific and technological base and in regulatory oversight.” “We remain committed to continuously supporting our countries to increase the production of critical medicines, as the region can meaningfully contribute to addressing the inequities we have seen to date,” Dr. Etienne said. She added that the recently launched PAHO Regional Platform will boost state-of-the-art pharmaceutical capacity, and “facilitate the transfer of technology to develop mRNA vaccines in the region while strengthening regulatory capacity and convergence to support these processes.” The WHO Emergency Use Listing is a procedure for assessing and listing vaccines, therapeutics and other medical tools to expedite their availability during a public health emergency. To date, eleven COVID-19 vaccines are listed under this mechanism. |